Overview
Aseptic products are contamination-free: they will not reproduce or create any kind of harmful living microorganisms (bacteria, viruses, and others). Sterile products are entirely free of all germs. The objective of aseptic processing is to maintain the sterility of an injectable product assembled from different components.
Containment and isolator technology
Overview
Different protection and containment systems can be proposed and customized for the protection of the product or the operator, or for both. The design of these cells can be highly customizable according to the regulations in force and the end-user requests.
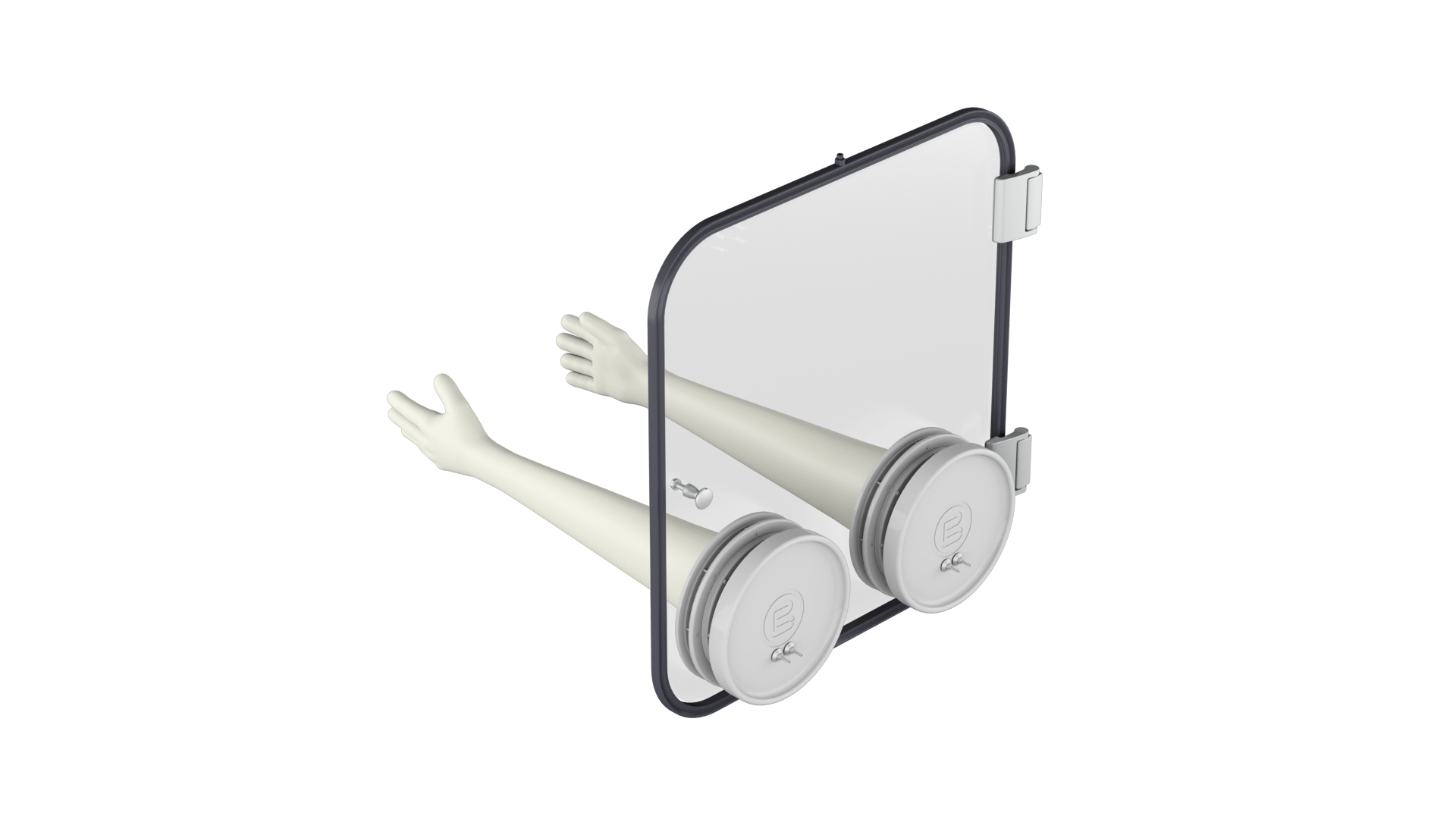
PBL-GT Glove Tester
The PBL-GT Glove Tester is an innovative system that ensures the highest standards of safety and performance in pharmaceutical applications. With precise pressure decay testing and customizable protocols, it detects even the smallest leaks, ensuring consistent results and enhanced operational efficiency. The PBL-GT Glove Tester combines cutting-edge software technology with unparalleled flexibility, delivering a fully integrable solution for glove management in both isolator and open RABS machines.
Read more
Characteristics
· Annex 1 Compliant
· CFR21 Part 11 Compliant
· Server-Client-Solution
· Active Directory Integration
· OPC UA/ SCADA
· Multiple testing of gloves
· Audit Trail
· GAMP 5
Key features
· PBL-GT (Glove Tester) can be integrated in the machine door or in stand-alone version
· Fast and reliable Pressure decay method Detects glove holes down to 30 microns
· Automated identification and detection of glove port
· Simultaneous tests of all gloves with overall process time in less than 20 minutes
· Customizable access levels and easy and reliable data management
· Easy and user-friendly operation via machine HMI
· Batch reports

Laf System
Laminar Air Flow (LAF) systems offer product protection from potential external contaminants.
This system is used both in laboratories with stand-alone cells and in production processes, such as formulation, filling, and closing of injectable and non-injectable pharmaceutical containers.
Product protection is achieved through a targeted air flow.
Read more
A low-turbulence pure air flow (laminar air flow) flows vertically into the working area of the cell and exits close to the ground – suspended particles are captured and removed in a controlled manner.
The air flow is constantly monitored by pressure sensors and digital anemometers, which will give an alarm every time the operating parameters exceed the pre-set limits.

Open/Closed RABS
O / C RABS (Open / Close Reduced Access Barrier System) are barrier systems used for operator and/or product protection requirements such as standard or special models. O / C RABS can be used for aseptic processes such as formulation, filling, and sealing of aseptic products.
Read more
Product protection is achieved through a barrier between the operator and the product. Product protection is created by the Laminar Air Flow System.
The air flow is constantly monitored by pressure sensors and digital anemometers, which will give an alarm every time the operating parameters exceed the pre-set limits.

Isolator Systems
Isolators for product and operator protection in close contact for sterile, aseptic, and toxic processes. The protection is obtained through a barrier (insulated glovebox) between the operator and the product. Product safety is guaranteed by the Laminar Air Flow.
Read more
Product safety is guaranteed by the Laminar The air flow is constantly monitored by pressure sensors and digital anemometers capable of generating an alarm should the operating parameters go out of the pre-set limit parameters.
The insertion of the materials takes place safely through the barrier transfer systems
The heart of the transfer system is the Alpha door with its secure interlocking which allows for totally secure connections and disconnections.
The system allows you to move material from one sterile zone to another through a non-sterile zone, with leak-proof reconnection and without risk.
Containers made of stainless steel or polyethylene allow for safe transfer in and out of a barrier system in various life science applications. The quick transfer system is based on the interaction of an Alpha part with a Beta part, each equipped with a door, a lock and a locking function.

Contact us

